Discharge Monitoring Report-Quality Assurance
We help NPDES permit holders navigate the requirements of the EPA’s annual DMR-QA study. When you partner with ERA, you gain access to experienced technical and customer service team members who provide expert guidance for every step of the study response process and ensure you have the right samples, at the right time.
Streamline your preparation for the DMR-QA study
From preparatory resources to product replacement and corrective action support, ERA offers industry leading guidance and tools to move you through the DMR-QA process efficiently.- Immediate notification of the EPA’s Section 308 letter when the study is announced
- Comprehensive explanation of PT process
- Help understanding and meeting regulatory requirements
- Free analysis tips and tricks webinars and resources
- Practice QC samples
- Corrective action parameters and support
- Access to ERA’s eDATA PT data management portal for ongoing program management
DMR-QA Quick links
Here are a few quick links to key guidance and forms to help you plan your process. Access even more from our comprehensive resources page.- DMR-QA 45 EPA Announcement Letter
- DMR-QA 45 Order Form
- DMR-QA 45 Planning Guide
- DMR-QA 45 Checklist
- Discharge Monitoring Report-Quality Assurance (DMR-QA) Program Explained
- eDATA How-to Video: Change your Permittee Contact
Get expert assistance for proficiency testing (PT) programs
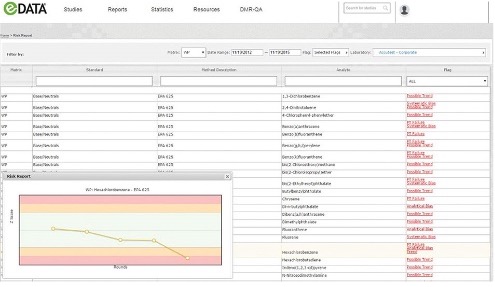
As your partner in defensible data, we provide you with tools to effectively manage your PT program, assess risk and evaluate trends over time. To make it even easier, ERA created eDATA™, an online PT data management portal that helps you improve quality longer term.Learn more about eDATA.
Access our order form and begin your DMR-QA journey.
Have questions? Contact our helpful customer service team.
Shop DMR-QA
Find Your Product

ERA DMR-QA webinars and videos help ensure pH, TSS and ammonia analysis success. Watch this sneak preview to learn about the topics we cover. Then, check out our webinar series.
DMR-QA 45 Study Dates
2025 Compliant Studies | Open | Close |
DMR-QA 45 | Jun 6 | Aug 29 |
WP-360 | Jan 21 | Mar 7 |
WP-361 | Feb 17 | Apr 3 |
WP-362 | Mar 10 | Apr 24 |
WP-363 | Apr 14 | May 29 |
WP-364 | May 12 | Jun 26 |
WP-365 | Jun 16 | Jul 31 |
WP-366 | Jul 14 | Aug 28 |
MilliporeSigma RTC Users
Did you use Sigma RTC in the past for your DMR-QA proficiency testing studies? Use our product comparison tool to find the equivalent ERA product
Find Your Product
Are you new to DMR-QA, or do you need a refresher on the process?
View DMR-QA educational videos and webinars
ERA DMR-QA webinars and videos help ensure pH, TSS and ammonia analysis success. Watch this sneak preview to learn about the topics we cover. Then, check out our webinar series.

